肥胖细胞型星形细胞瘤的MRI表现探讨
吴玉珍,钟群,林金贵,吴慧敏
南京军区福州总医院第476临床部医学影像科,福建 福州 350025
[摘 要]目的初步探讨肥胖细胞型星形细胞瘤(Gemistocytic Astrocytoma,GemA)的MRI表现及功能成像特点,以提高对该病的认识及诊断水平。方法回顾性分析2010年10月~2015年12月经手术及病理证实的13例GemA患者的临床及影像资料,所有患者均行MRI平扫、增强,其中9例加扫磁共振波普(Magnetic Resonance Spectroscopy,MRS)、弥散张量成像(Diffusion Tensor Imaging,DTI)、动脉自旋标记技术(Arterial Spin Labeling,ASL)及灌注加权成像(Perfusion Weighted Imaging,PWI)成像,1例加扫磁敏感加权成像(Susceptibility Weighted Imaging,SWI)序列,分析总结其MRI影像特征。结果13例患者均为单发病灶,且均发生于幕上,其中发生于额叶10例,颞叶2例,顶叶1例;8例病灶局限于单脑叶,5例累及多个脑叶,并通过胼胝体侵犯对侧脑组织;边界不清8例,边界清楚5例;肿瘤内部有大小不等囊变成分7例。肿瘤的实性成分在T1WI呈稍低信号,T2WI呈稍高信号,DWI呈稍高信号,增强后肿瘤实性成分多呈中度及以上不均匀强化;MRS表现为Cho峰升高,NAA峰减低,Cr峰基本稳定;ASL及使用PWI均呈低灌注,DTI显示患侧大脑皮质纤维束明显较对侧稀疏。结论GemA的MRI表现为幕上单发,额叶多见,多边界不清且内部常伴囊变,并累及胼胝体侵犯对侧脑叶,增强扫描肿块实性成分多中度及以上不均匀强化,结合MRS、DTI、ASL、SWI及PWI,可对本病提供诊断帮助。
[关键词]肥胖细胞;星形细胞瘤;磁共振成像;磁共振波谱成像;弥散加权成像
引言
肥胖细胞型星形细胞瘤(Gemistocytic Astrocytoma,GemA)是一种较为罕见的颅内肿瘤,其为II级弥漫性星形细胞瘤的亚型[1],手术是其主要的治疗方法,但术后极易复发,且复发后恶性程度更高[2],预后较差。GemA比其它亚型弥漫性星形细胞瘤更容易向高级别的星形细胞瘤方向发展[3]。术前准确的影像学诊断对临床手术方案的制定及预后评估具有重要价值。本研究回顾分析了13例经手术及病理证实的GemA,探讨了其影像学表现征象,以期提高对其诊断准确性,并对临床治疗提供较为可靠的影像学依据。
1 材料与方法
1.1 一般资料
收集本院2010年10月~2015年12月经手术及病理证实的GemA 13例,其中男8例,女5例,年龄25~76岁,中位年龄45.5岁。临床症状以进行性加重头晕、头痛为主诉10例,2例晕厥、肢体乏力/麻木,1例言语不清、癫痫。
1.2 检查方法及扫描参数
采用Siemens Magnetom Trio 3.0T MR成像系统,8通道相控阵颅脑专用线圈。患者按标准体位摆位,头颅两侧加海绵垫以固定头部。
扫描序列及参数:① T2WI矢状位3D扫描,TR 3200 ms,TE 407 ms,FOV 250 mm×250 mm,FA 120°,矩阵 256×256,层厚1.1 mm,层间距0,NEX 1,重建轴位及冠状位图像,用于波谱定位及弥散张量成像(Diffusion Tensor Imaging,DTI)后处理;② T1WI轴位扫描,TR 1829 ms,TE 25 ms,FOV 230×188,矩阵320×218,层厚5 mm,层间距0.5 mm;③ Flair轴位扫描,TR 7000 ms,TE 100 ms,层厚5 mm,层间距0.5 mm,FOV 230 mm×188 mm,矩阵256×192;④ 弥散加权成像(Diffusion Weighted Imaging,DWI)采用单次激发平面回波序列,TR 5100 ms,TE 90 ms,层厚5 mm,层间距0.5 mm,FOV 240 mm×240 mm,矩阵192×192;⑤ 磁共振波普(Magnetic Resonance Spectroscopy,MRS)扫描采用3D多体素1H-MRS,TR 900 ms,TE 145 ms,Average 6;⑥DTI扫描TR 6900 ms,TE 93 ms,扩散方向12个;⑦ 动脉自旋标记技术(Arterial Spin Labeling,ASL)扫描TR 2300 ms,TE 13 ms,FA 90°;⑧ 磁敏感加权成像(Susceptibility Weighted Imaging,SWI)扫描 TR27 ms,TE20 ms,层厚1.50 mm;⑨ 灌注加权成像(Perfusion Weighted Imaging,PWI)TR2000 ms,TE 45 ms,FA 90°,对比剂为钆喷酸葡胺(Gd-DTPA),剂量为0.2 mL/kg,以2~3 mL/s的流率经右肘前静脉注射。与注射同步灌注扫描,后行轴冠矢三平面增强扫描。
2 结果
2.1 MRI影像学表现
肿瘤的发生部位及大小:所有病灶均发生在幕上脑实质且均为单发。其中,位于额叶10例,颞叶2例,顶叶1例;13例中发生于单个脑叶者8例,累及多个脑叶者5例,并通过胼胝体侵犯对侧脑组织。病灶最小径约2.7 cm,最大径约7.2 cm,平均直径约4.1 cm。
肿瘤形态及MRI表现:肿瘤沿矢状径生长,贴近纵裂池,累及皮髓质,占据大脑半球1/2左右,肿瘤实性部分在T1WI上呈等或稍低信号,在T2WI上呈等或稍高信号,在DWI上呈稍高为主;8例肿瘤周围均伴有轻度水肿,其余未见明显水肿征象;8例呈弥漫性生长,边界不清;5例边界清楚,内部见大小不等的囊变区,在T1WI呈低信号,在T2WI呈高信号,其中1例表现为大囊结节型,实性结节位于肿瘤的脑膜面侧。MRS特征:对其中9例实性成分较多肿瘤行MRS波谱分析,实性病灶感兴趣区Cho峰升高,NAA峰减低,Cr峰基本稳定。ASL及PWI呈低灌注,DTI显示患侧大脑皮质纤维束明显较对侧稀疏(图1)。增强扫描肿瘤的实性成分呈不均匀中度及以上强化9例,3例增强后轻度强化,1例无明显强化。
2.2 手术所见及病理结果
术中可见肿瘤呈灰白色,肿瘤质地较软,血供一般,与周围正常脑组织界限不清。术后病理免疫组化结果显示均为弥漫性肥胖细胞型星形细胞瘤(WHO Ⅱ级),其中4例显示局灶形态学达间变型(Ⅲ级),1例20%为少突胶质细胞瘤,1例并发脑膜转移。
3 讨论
根据WHO 2007年中枢神经系统肿瘤的最新分类,II级弥漫性星形细胞瘤分为三种亚型:纤维型(Fibrillary)、肥胖细胞型(Gemistocytic)及原浆型(Protoplasmic)。最常见的亚型是纤维型,约占65.2%;肥胖型其次,约占29%;原浆型约占5.8%[4]。病理学上GemA的诊断标准为肥胖细胞占比高于20%,但很少超过50%[5]。特征性表现是肥胖细胞体积肥大,细胞排列紧密,胞质丰富,嗜酸性,核偏位,多呈圆形、卵圆形,核分裂少见。肥胖细胞呈神经胶质酸性蛋白免疫蛋白高表达。GemA比其它亚型弥漫性星形细胞瘤更容易向高级别的星形细胞瘤方向发展,肿瘤生长相对迅速,生存期较其它弥漫性星形细胞瘤短,多在一年内。本组随访3例肿瘤术后复发,病理证实为胶质母细胞瘤(IV级)。GemA平均发病年龄约46.8岁左右,男女比例为1.42~3.50[4]。本组患者平均发病年龄在45.5岁,男女比为1.6,与文献基本一致。
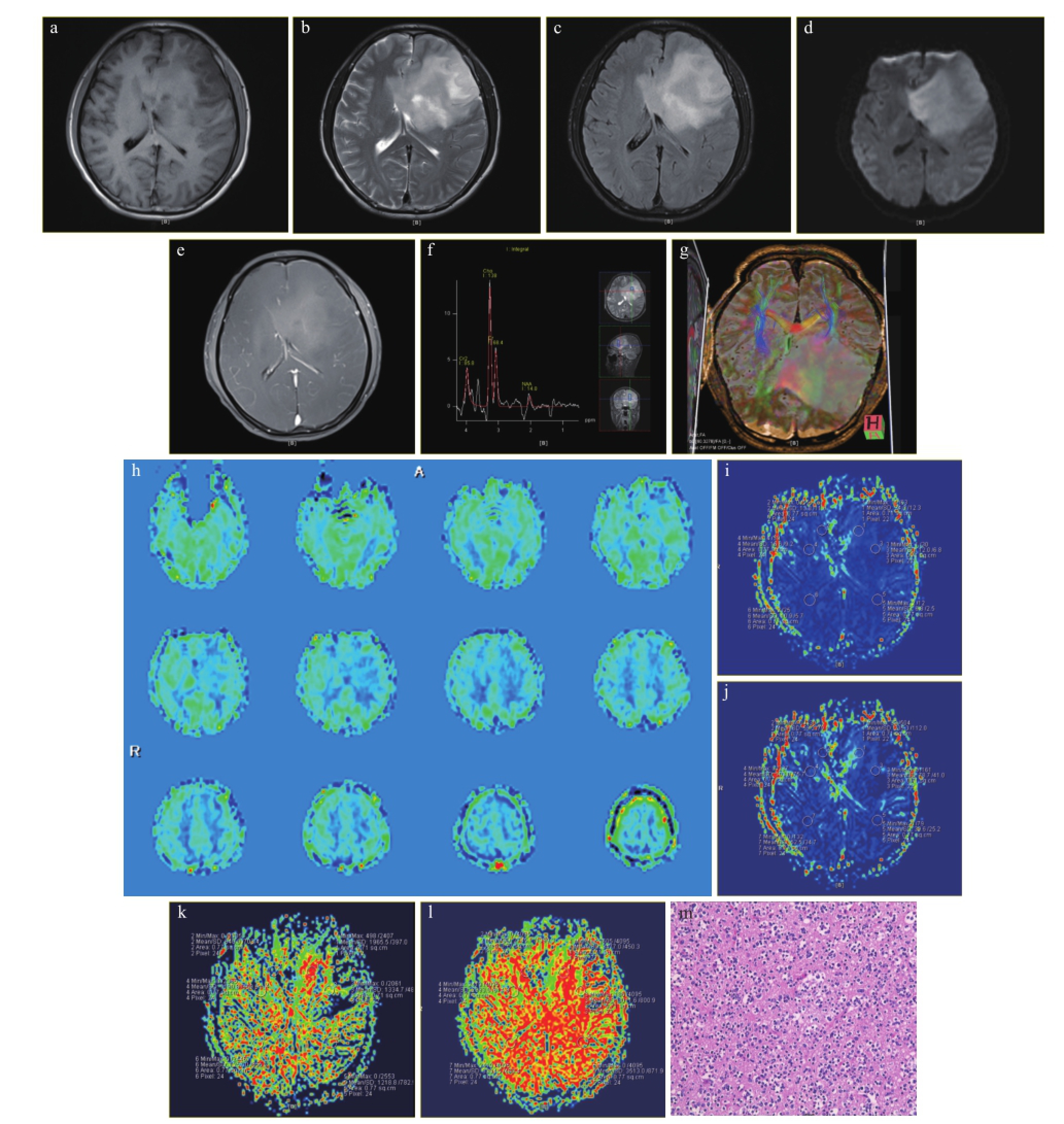
图1 左侧额颞叶GemA的MRI影像表现
注:a.T1WI呈等信号;b.T2WI呈稍高信号;c.Flair呈稍高信号;d.DWI呈稍高信号,周围未见明显水肿带;e.增强扫描呈轻度强化;f.MRS示病灶区Cho峰升高,NAA峰减低;g.DTI示左侧额叶病灶区纤维束较对侧稀疏减少;h.ASL示病灶区低灌注,PWI示病灶区低灌注;i~j.CBFi、CBV病侧较对侧通过率减低;k~l.MTT、TTP延长,病理显示细胞体积肥大;m.细胞排列紧密,胞质丰富,嗜酸性,核偏位。
GemA多发于幕上,最好发于额叶,其次为颞叶、顶叶及枕叶;迄今未有小脑部位的报道。本组13例均发生在幕上,其中累及额叶10例(76%),累及颞叶2例(15%),累及顶叶1例(7%),与文献报道[5-6]趋于一致。GemA大多单发,一般表现为弥漫浸润性生长,边界不清,瘤周水肿多为轻度,占位效应较轻,此表现符合II级弥漫性星形细胞瘤介于良恶性之间的性质。肿瘤生长缓慢,病程较长,因此发现时体积常较大,本组病例中最大直径约7.2 cm。本组5例(38%)磁共振表现累及多个脑叶,通过胼胝体侵犯对侧脑组织;笔者认为此为GemA具有一定的恶性潜质的一种特征性表现;而文献[5]认为特殊表现为大囊结节型,实性结节位于肿瘤的近脑膜面,影像学表现特点与神经元肿瘤类似;本组中仅1例具有此表现。肿瘤的实性成分在T1WI呈等或稍低信号,在T2WI呈等或稍高信号,在DWI呈稍高信号为主;肿瘤内部信号欠均匀,易囊变,出血少见,本组1例瘤内出血加扫SWI,显示低信号出血灶;肿瘤内部钙化罕见,也有可能MRI不显示细小钙化有关。MRS显示实性部分病灶感兴趣区Cho峰升高,NAA峰减低,Cr峰基本稳定;ASL及PWI呈低灌注,DTI显示患侧大脑皮质纤维束明显较对侧稀疏[7-12]。增强后肿瘤实性成分呈中度及以上强化是本病案的另一特征性表现,少部分肿瘤可轻度强化或不强化。一般II级弥漫性星形细胞瘤多表现轻度强化或不强化,而明显强化的区域常提示向间变型或胶质母细胞瘤分化。因此GemA的强化方式易被误诊为恶性肿瘤。
GemA常与以下几种疾病相鉴别:① 纤维型星形细胞瘤,WHOⅡ级,呈弥漫性生长,边界不清,但极少通过胼胝体侵犯对侧脑组织,增强后肿瘤多为轻度强化或不强化,而GemA的实性成分多中等以上强化;② 淋巴瘤[13],好发于大脑深部近中线脑白质区,多发病变易累及胼胝体。瘤内出血、囊变极其少见;瘤周水肿轻,MRS上Lip明显升高及“缺口征”强化具有一定特异性,根据其增强、ASL及PWI的低强化高灌注特性很容易鉴别;③ 胶质母细胞瘤[14-18],沿胼胝体侵犯至对侧脑组织形成蝴蝶状改变,周围水肿及占位效应更明显,增强后呈花环状强化。
综上所述,GemA具有特征性的MRI表现,除常规平扫及增强外,结合MRS、DTI、ASL、SWI及PWI,对于提高本病的诊断正确率有重要的意义。此外,本研究因病例较少,提取数据有限,无法提供完全确定性诊断,需要以后在临床工作中积累更多病例来综合评估。
[参考文献]
[1] Louis DN,Ohgaki H,Wiestler OD,et al.The 2007 WHO classif i cation of tumours of the central nervous system[J].Acta Neuropathol,2007,114(2):97-109.
[2] Reis RM,Hara A,Kleihues P,et al.Genetic evidence of the neoplastic nature of gemistocytes in astrocymas[J].Acta Neuropathol,2001, 102(5):422-425.
[3] Tove LL,Hansson HA,Stein S,et al.Prognostic value of histologicalfeatures in diffuse astrocytomas WHO grade II[J]. Int J Clin Exp Patho,2012,5:152.
[4] Babu R,Bagley JH,Park JG,et al.Low-grade astrocytomas: the prog-nostic value of fibrillary,gemistocytic,and protoplasmic tumor histol-ogy: Clinical article[J].J Neurosurg, 2013,119:434-441.
[5] 李建瑞,王朋,张军,等.肥胖型星形细胞瘤的影像学表现与病理研究[J].医学影像学杂志,2015,25(6):943-947.
[6] 李建瑞,程晓青,陆珍凤,等.肥胖细胞型星形细胞瘤的MRI表现:8例病例分析[J].临床放射学杂志,2015,34(7):1044-1047.
[7] Kallenberg K,Goldmann T,Menke J,et al.Glioma infiltration of the corpus callosum: early signs detected by DTI[J].J Neurooncol, 2013,112(2):217-222.
[8] Coban G,Mohan S,Kural F,et al.Prognostic Value of Dynamic Susceptibility Contrast-Enhanced and Diffusion-Weighted MR Imaging in Patients with Glioblastomas[J].Am J Neuroradiol, 2015,36(7):1247-1252.
[9] 黄丹江,孙胜军,李滢.下丘脑/视交叉区毛细胞黏液样型与毛细胞型星形细胞瘤的MRI征象对比[J].实用放射学杂志,2015,12(7):1078-1081.
[10] 张蕊,彭晓刚,崔丽华,等.磁共振在多形性黄色星形细胞瘤诊断中的价值[J].肿瘤学杂志,2015,21(2):157-159.
[11] Zou QG,Xu HB,Liu F,et al.In the assessment of supratentorial glioma grade:The combined role of multivoxel proton MR spectroscopy and diffusion tensor imaging[J].Clin Radiol,2011,66(10):953-960.
[12] 刘畅,季学兵,王堂娟.脑胶质瘤瘤体及瘤周MRS、ADC值与p53的相关性研究[J].中国CT和MRI杂志,2016,14(11):25-27.
[13] 张海捷,张雪林.原发性中枢神经系统淋巴瘤的MRI表现与病理学对照研究[J].临床放射学杂志,2010,29(2):148-151.
[14] Yamasaki F,Takayasu T,Nosaka R,et al.Magnetic resonance spectroscopy detection of high lipid levels in intraaxial tumors without central necrosis: a characteristic of malignant lymphoma[J]. J Neurosurg,2015,122(6):1370-1379.
[15] Ali S,Joseph NM,Perry A,et al.Apparent diffusion coefficient in glioblastoma with PNET-like components, a GBMvariant[J]. J Neuro-oncol,2014,119(2):353-360.
[16] 陈传亮,白岩,王梅云,等.三维伪连续性动脉自旋标记磁共振灌注成像联合扩散加权成像在脑胶质瘤分级中的价值[J].中国医学计算机成像杂志,2015,21(5):426-430.
[17] 于同刚,吴丽琼,戴嘉中,等.MRS在星形细胞肿瘤术后放疗中的价值: Cho/Cr的变化及意义[J].中国医学计算机成像杂志, 2015,21(2):101-104.
[18] 王国华,石艳艳,宋修峰,等.肿瘤周围的1H-MRS在常见颅脑肿瘤鉴别诊断中的价值[J].医学影像学杂志,2015,12(6):958-961.
本文编辑 聂孝楠
Investigation of Magnetic Resonance Imaging Manifestations of Gemistocytic Astrocytoma
WU Yu-zhen, ZHONG Qun, LIN Jin-gui, WU Hui-min
Department of Medical Imaging, Fuzhou General Hospital of Nanjing Military Region 476thHospital Medical, Fuzhou Fujian 350025, China
Abstract:ObjectiveTo preliminarily investigate the MRI manifestations and imaging features of gemistocytic astrocytoma (GemA) so as to improve the level of recognition and diagnosis of the disease.MethodsThe clinical and imaging data of 13 patients with GemA, diagnosed via surgery as well as pathology from October 2010 to December 2015, were reviewed retrospectively. All patients were performed by both plain and enhanced MRI scanning. 9 cases of them still were performed with magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI), arterial spin labeling (ASL) and perfusion weighted imaging (PWI) scanning, along with 1 case through susceptibility weighted imaging (SWI) sequence. Then the related MRI imaging features were analyzed and summarized.Results13 GemA patients were all solitary lesions and located at supratentorial sites, with 10 cases located in the frontal lobe, 2 cases in temporal lobe, 1 case in parietal lobe. Of these 13 cases, 8 cases were conf i ned to single lobe lesion, 5 cases were involved in multiple lobe which invaded the opposite brain tissue through corpus callosum; 8 cases had unclear boundary, the other 5 cases presented with clear boundary; 7 cases showed multiple and various sizes of cystic lesions in solid part. The solid portions of the tumor demonstrated slightly hypo intense on T1WI, slightly hyper intense on T2WI and DWI, and obviously uneven enhancement in moderate or above level; MRS characterized that Cho (choline) peak increased, NAA (N-acetyl aspartic acid) peak decreased and Cr (creatine) peak was basically stable; ASL and PWI presented hypoperfusion, DTI showed lateral cortical fi ber bundle was sparser obviously than contralateral side.ConclusionMRI manifestations of GemA were mainly single, located in the frontal lobe, much unclear boundary with cystic change inside, invading the opposite tissues through corpus callosum, and uneven enhanced in moderate or above level to solid elements of tumor. What’s more, MRS, DTI, ASL, SWI and PWI could be combined with MRI to help diagnose the disease.
Key words:gemistocyte; astrocytoma; MRI; magnetic resonance spectroscopy; diffusion weighted imaging
[中图分类号]R445.2;R322.8
[文献标识码]B
doi:10.3969/j.issn.1674-1633.2017.04.022
[文章编号]1674-1633(2017)04-0083-04
收稿日期:9
收稿日期:2016-06-15 修回日期:2016-07-26
基金项目:南京军区联勤部课题(12MA097)。
通讯作者:钟群,副主任医师,研究方向为医学影像技术。
通讯作者邮箱:zhongqun85@126.com