Polymeric Nerve Conduits with Contact Guidance Cues Used in Nerve Repair
G.DAI1,X.NIU1,J.YIN2
1.Department of Medical Engineering,Ningbo First Hospital,Ningbo Zhejiang 315000 China;2.School of Mechanical Engineering,Zhejiang University,Hangzhou Zhejiang 310058 China
Abstract:In the modern life,the nerve injury frequently happens due to mechanical,chemical or thermal accidents.In the trivial injuries,the peripheral nerves can regenerate on their own;however,in most of the cases the clinical treatments are required,where relatively large nerve injury gaps are formed.Currently,the nerve repair can be accomplished by direct suture when the injury gap is not too large;while the autologous nerve graft working as the gold standard of peripheral nerve injury treatment for nerve injuries with larger gaps.However,the direct suture is limited by heavy tension at the suture sites,and the autologous nerve graft also has the drawbacks of donor site morbidity and insuffcient donor tissue.Recently,artifcial nerve conduits have been developed as an alternative for clinical nerve repair to overcome the limitations associated with the above treatments.In order to further improve the effciency of nerve conduits,various guidance cues are incorporated,including physical cues,biochemical signals,as well as support cells.First,this paper reviewed the contact guidance cues applied in nerve conduits,such as lumen fllers,multi-channels and micro-patterns on the inner surface.Then,the paper focused on the polymeric nerve conduits with micro inner grooves.The polymeric nerve conduits were fabricated using the phase inversion-based fber spinning techniques.The smart spinneret with grooved die was designed in the spinning platform,while different spinning conditions,including flow rates,air-gap distances,and polymer concentrations,were adjusted to investigate the infuence of fabrication conditions on the geometry of nerve conduits.The inner groove size in the nerve conduits can be precisely controlled in our hollow fber spinning process,which can work as the effcient contact guidance cue for nerve regeneration.
Key words:nerve injury;nerve repair;polymeric nerve conduits;contact guidance cues;fber spinning
0 INTRODUCTION
The human nervous system can be divided into the central nervous system and the peripheral nervous system.The peripheral nervous system includes the cranial nerves arising from the brain,the spinal nerves arising from the spinal cord,and sensory nerve cell bodies and their processes.With the development of modern life,peripheral nerves are commonly exposed to physical injuries,such as sports injuries,construction accidents,natural disaster,war damage,and other trauma like fractures.Meanwhile,nerve injury can also be caused by biochemical reasons such as diabetes,toxicity,infection,and other congenital diseases.
Among all kinds of nerve injuries,the most serious situation generally is nerve transaction;while the data shows peripheral nerve trauma caused about 5% of all open woundsin the extremities,most wounds had formed nerve gap[1],which generally leads to disability of individual’s function at work and in daily life.In the United States,approximately 360,000 individuals suffer from upper extremity paralysis each year,and over 200,000 of these patients undergo surgical operation each year[2].However,the outcome following clinical nerve injury repair is far from satisfactory that less than 50% of individuals that undergo surgical repair regain useful function[3],and many patients suffer from permanent or long-term disability.Although,it costs more than $150 billion in health care costs in the United States each year[4],few experimental therapies have been applied to clinical.Thus,a critical need remains for the development of novel and effective bridging strategies for peripheral nerve regeneration.
Although,peripheral nerves can regenerate on their own over relatively short distances,microsurgical repair is essential in the cases of larger injury gaps.Currently,treatment for peripheral nerve injury typically consists of either direct surgical reconnection of the damaged nerve ends or the use of an autograft (Figure 1).Direct surgical reconnection (also known as end-to-end suturing,end–end repair,end-to-end neurorrhaphy or end-to-end coaptation) achieves optimal results of nerve regeneration in the cases of short nerve gap and minimal tissue damage.However,the successful end-to-end suturing of damaged nerve requires tension-free suturing of the injury site,which is limited to nerve gaps shorter than 5 mm[5].Autologous nerve graft (autograft) is used for clinical repair of relative large nerve gap.In this treatment,a comparable nerve is frst removed from another part of the patient's body,and then used to bridge the gap and connect the two ends of the severed nerve.Currently,autologous nerve graft plays as the gold standard of nerve repair[6],but the use of autologous nerve graft has a number of disadvantages,including donor site morbidity,the requirement for a second surgical site,a very limited supply,donor site mismatch and the possibility of painful neuroma formation and scarring[7].Moreover,autologous nerve graft also requires secondary removal of degenerated axons and myelin by the host from the graft itself,increasing the healing time[8].Because of the defects existed in direct suture and autologous nerve graft,there is a urgent clinical need to fnd an alternative approach that matches or exceeds the performance of above methods.
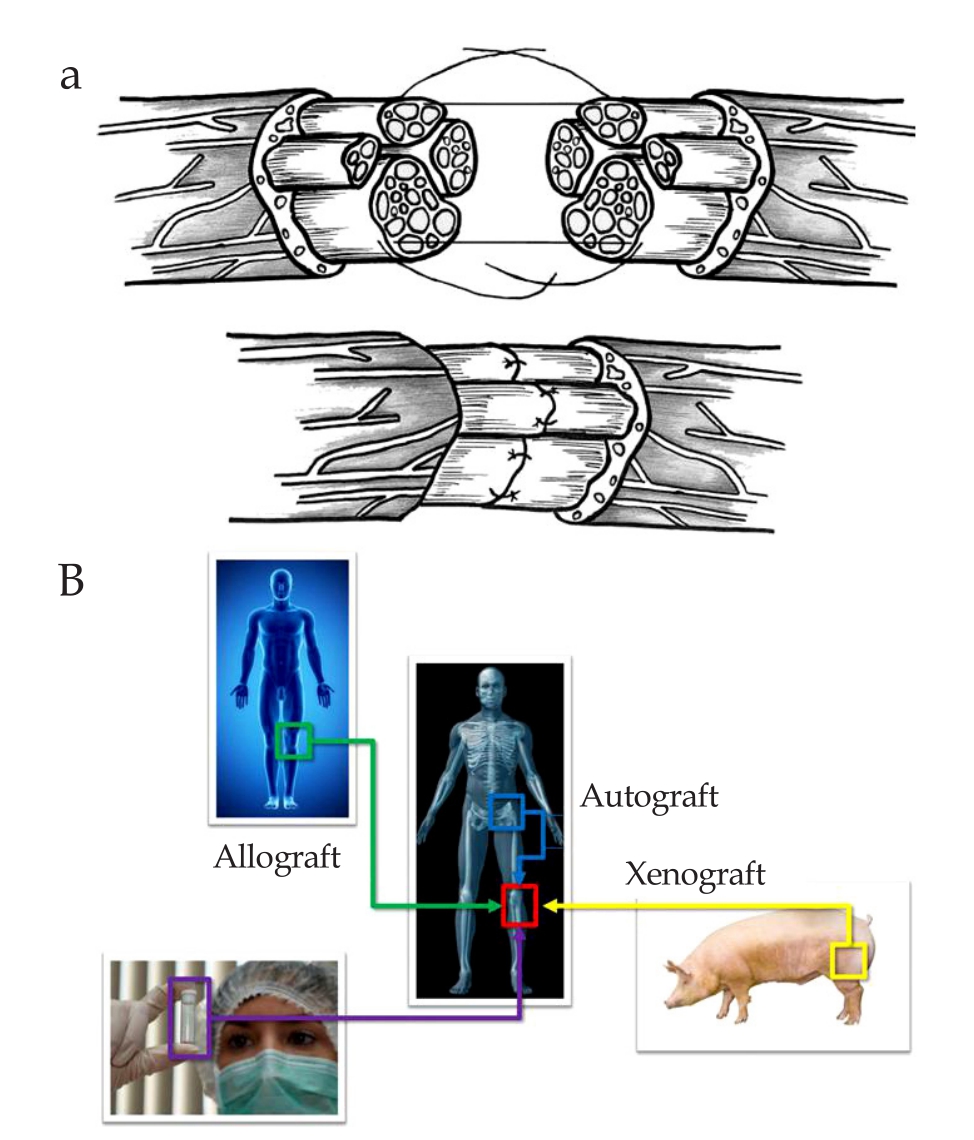
Figure 1 Treatment for peripheral nerve injury typically consists of either direct surgical reconnection of the damaged nerve ends or the use of an autograft.A: Direct surgical reconnection of nerve gap,adapted from Ref[2];B: Different type of nerve graft,adapted from http://www.graftys.com/en/faq/.
1 NERVE CONDUIT AND GUIDANCE CUES
1.1 Nerve conduit
Tissue engineering,an emerging multidisciplinary field,has grown at a significant rate in recent years.The emerging feld of tissue engineering offers a new prospect neuroscientists and surgeons for treatment strategies of injured nerves.As most of tissue engineered products,tissue scaffolds have been designed to serve as templates that support the regeneration of injured nerves,which is termed as nerve conduit.A typical nerve conduit is a tubular structure designed to bridge the gap of a sectioned nerve,protect the nerve from the surrounding tissue,e.g.,scar formation,and guide the regenerating axons into the distal nerve stump[9].
The functions and advantages of the nerve conduit can be summarized as[10-12]:
(1) Maintaining adequate mechanical support for the regenerating nerve fbers;
(2) Providing a conduit channel for the diffusion of neurotropic and neurotrophic factors secreted by the damaged nerve stump and a conduit wall for the exchange of nutrients and waste products;
(3) Obviation of the infltration of fbrous scar tissue that hinders axonal regeneration;
(4) Creating an optimal microenvironment to accumulate and release the exogenous and endogenous biochemical effectsfor nerve regeneration.
The basic structure of nerve conduits is a hollow cylindrical tube with a single lumen;however considerable efforts have been made to optimize their nerve repair effciency.An appropriate nerve conduit must be biodegradable and biocompatibility,such as low inflammatory and cytotoxic responses.In addition,the mechanical properties of the nerve conduit must guarantee that it does not collapse during the patient's movements but at the same time is suffciently elastic to avoid tensions in the lesion site.Furthermore,nerve conduits have to be permeable to the entry of nutrients into the conduit lumen but presenting the necessary barrier to prevent the infltration of unwanted tissues into the conduit from outside[13].Various techniques have been involved in the design of an ideal nerve conduit for peripheral nerve repair,and most of these modifcations are aimed at increasing the nerve gap that can be bridged[14].
In order to improve the axonal regeneration through the nerve conduit across long lesion gaps,additional growthpromoting factors,termed as guidance cues,are introduced into nerve conduits.
1.2 Biochemical guidance cues
The guidance cues may be broadly classified into biochemical and physical guidance cues.In terms of biochemical signaling,growth/neurotrophic factors,cells,nucleic acids and extracellular matrix (ECM) molecules such as collagen,laminin and fbronectin are often involved.
Neurotrophic factors enhance nerve functional regeneration controlling the survival,migration,proliferation and differentiation of various neural cell types,and supporting axonal growth.In particular,nerve growth factor (NGF),neurotrophin-3 (NT-3),glial cell-line derived neurotrophic factor (GDNF) and fbroblast growth factors (acidic and basic,aFGF and bFGF) are among the most common choices[15].
Cell transplantation and its incorporation into nerve conduit designs are alternative strategies to neurotrophic factor delivery.Schwann cells migration and proliferation play the essential role in supporting axonal outgrowth following peripheral nerve injuries.Since Schwann cells produce various ECM molecules,release bioactive factors,and express many cell adhesion molecules and receptors in the injured region,Schwann cells offer a highly preferred substrate for axon migration,promote blood vessel formation,and form longitudinally oriented strands (bands of Büngner) serving as a guiding rail for regenerating axons.Thus,Schwann cells are most favored cellular components for peripheral nerve regeneration[15].
1.3 Physical guidance cues
The main form of the physical guidance is the three dimensional scaffold with different structures in the lumen of the nerve conduit,such as filaments,multichannel structures and grooves shown in Figure 2.
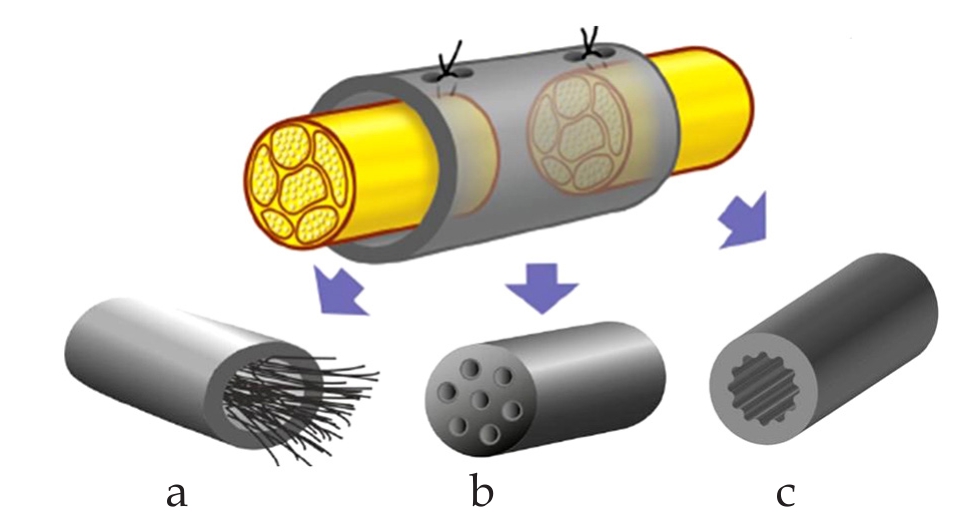
Figure 2 Physical guidance cues.a: Intraluminal guidance structures;b: Multi-channel conduits;c: Micro-grooved luminal designs.
The addition of structural intraluminal guidance cues may act as a replacement for the unformed or incomplete fibrin cable,or act as an additional anchor for its formation.A number of similar studies were carried out using different intraluminal guidance structures include gels,sponges,films,filaments and fibers[16].The use of a multi-channel conduit is also an alternative to conventional nerve conduits,which was closely imitating native nerve’s architecture.The multi-channel conduits have been manufactured using a foam-processing technique,where a fve channel conduit was then successfully used to bridge a short 7 mm rat sciatic nerve gap[17].
Since the surface texture in micrometric scale has great influence on cell alignment,which plays an important role during the regeneration of the damaged nerves.Various fabrication methods have been applied.Rutkowski et al[18]fabricated the conduits with micropatterned by inserting micropatterned biodegradable flms into smooth nerve conduit.Hsu et al[19,20]made biodegradable chitosan and PLA films with microgrooves (20/20/3 μm) by photolithography and etching process using the negative photoresist.Then,the micropatterned substrates were rolled into conduits,and the edges were adhered by a small amount of solvent.Long et al[21]fabricated semipermeable hollow fiber membranes with a textured inner surface by the wet phase-inversion process,but the grooved inner texture was caused by process-induced instability.Thus,the groove size and geometry was irregular and not controllable.
2 FABRICATION OF INNER GROOVE NERVE CONDUITS
In this work,a fabrication method of nerve conduit with controlled inner grooves was presented.As a typical hollow fiber membrane,the polymeric nerve conduit can be manufactured by immersion precipitation-based phase inversion method.Phase inversion is a process whereby a polymer is transformed from a liquid to a solid state,while the majority of the phase inversion membranes are prepared by immersion precipitation[22].In this method,the polymer is dissolved in a suitable solvent,forming the polymer solution,and then the polymer solution is immersed into a coagulation bath containing a nonsolvent.The polymer solution decomposes into one polymer rich phase and one polymer lean phase due to the diffusion and convection-induced mass transfer between the solvent and nonsolvent.After phase separation,the polymer solution solidifes and the polymer rich phase develops into a polymer dense matrix;while the polymer lean phase develops into macrovoids[23].
2.1 Fabrication setup
The spinneret utilized in this work is shown in Figure 3.There are two inlets on the spinneret that one is for polymer solution and the other for nonsolvent.During the fabrication process,the polymer solution is co-extruded with an inner nonsolvent through the spinneret,and then both flows pass through an air gap to enter a coagulation bath.There are also two tubes at the spinneret outlet,where nonsolvent is extruded through inner tube and polymer solution goes through the space between inner and out tube.It can be noticed that a grooved inner tube was implemented at the outlet of spinneret.Thus,the polymer solution was restricted to a certain grooved geometry within the spinneret.Since the phase separation takes place once polymer solution contacts with nonsolvent at the outlet,the polymer solution solidifes with the grooved geometry kept on the inner surface of nerve conduit.In the fabrication process,the flow rates of both polymer solution and nonsolvent were controlled by micro-fow pumps (Longer LSP01-1A,China).
2.2 Fabrication of nerve conduit
In this work,the Polyacrylonitrile (PAN,Mw=150000 Da) obtained from Sigma-Aldrich (USA) was used as the polymer material,while dimethyl sulfoxide (DMSO) provided by Sigma-Aldrich (USA) was used as solvent.The pure deionized water was used as non-solvent in fabrication and coagulant in coagulation bath.The polymer solution was prepared by dissolving PAN in DMSO with stirring for 8 h,then polymer solution was kept rest for 24 h to completely dissolve PAN in DMSO and release the air bubbles in the polymer solution.

Figure 3 Spinneret and grooved inner tube for nerve conduit fabrication.
The geometry of grooved hollow fiber was found to be sensitive to operation conditions during fabrication process[24].Therefore,the polymer solution fow rate was pumped at 0.5-3 mL/min,while the fow rate of nonsolvent was controlled at 0.3-2.5 mL/min.The air gap distance between spinneret outlet and the top surface of coagulation bath was kept as 2 cm.The polymer concentration varied from 7% to 9% (w/w).
2.3 Fabrication results
The nerve conduits with grooves in the longitudinal direction on the inner surface were obtained by the immersion precipitation-based phase inversion process.As shown in Figure 4,the groove geometry is as regular as die geometry.It was found that both nerve conduit size and groove geometry are sensitive to the fabrication conditions.For the nerve conduit diameters,the inner nonsolvent fow rate increases both inner and outer diameters,which decrease with air gap distance.The groove geometry can be characterized by the groove height and width between two groove bottoms.It was obtained that the groove width increases with nonsolvent fow rate and decreases with polymer solution flow rate;while the groove height decrease with nonsolvent fow rate.
Besides the nerve conduits with regular grooves,the irregular deformed confguration of nerve conduit cross section was also obtained within a certain fabrication condition.The similar observation was also reported in our previous study of nerve conduit fabrication with smooth spinneret.It isbelieved that the irregularity is caused by the process-induced instability[24].Thus,the fabrication conditions need to be carefully controlled,eliminating the instability and precisely regulating the groove size.
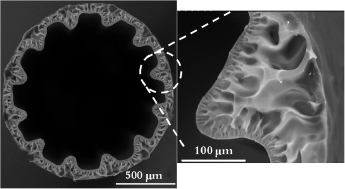
Figure 4 Scanning electron microscopy of the cross section of nerve conduit with groove inner surface.
3 CONCLUSION
In this work,the clinical repair approaches of peripheral nerve injury were reviewed,while it was found nerve conduits offer an alternative and improved treatment.The guidance cues used to improve the axonal regeneration were introduced,and our research was focused on the grooved inner surface of nerve conduit as the physical guidance cue.
The paper reports fabricating polymeric nerve conduits with a textured inner surface using immersion precipitationbased phase inversion method.The novel spinneret with grooved die was designed,and proved to be the effective device for fabrication of grooved nerve conduit.This patterned conduit is expected to have improved performance than smooth conduit on nerve regeneration.
4 ACKNOWLEDGMENTS
This study was jointly supported by National Natural Science Foundation of China (NSFC,No.11402056),and the Shanghai Committee of Science and Technology,China (No.14ZR1403300).
[REFERENCES]
[1]Ijkema-Paassen J,Jansen K,Gramsbergen A,et al.Transection of peripheral nerves,bridging strategies and effect evaluation[J].Biomaterials,2004,25:1583-1592.
[2]Hill PS.Regeneration of peripheral nerves using neuroinductive biomaterial scaffolds[D].Winston-Salem Wake Forest University,2009.
[3]Lee SK,Wolfe SW.Peripheral nerve injury and repair[J].J Am Acad Orthop Surg,2000,8:243-252.
[4]Praemer A,Furner S,Rice DP.Musculoskeletal conditions in the United States[M].USA:American Academy of Orthopaedic Surgeons,1999.
[5]Belkas SJ,Shoichet SM,Midha R.Peripheral nerve regeneration through guidance tubes[J].Neurol Res,2004,26:151-160.
[6]Bozkurt A,Deumens R,Beckmann C,et al.In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels[J].Biomaterials,2009,30:169-179.
[7]Alluin O,Wittmann C,Marqueste T,et al.Functional recovery after peripheral nerve injury and implantation of a collagen guide[J].Biomaterials,2009,30:363-373.
[8]Pollard JD,Fitzpatrick L.An ultrastructural comparison of peripheral nerve allografts and autografts[J].Acta Neuropathologica,1973,23:152-165.
[9]Pfister LA,Papaloios M,Merkle HP,et al.Nerve conduits and growth factor delivery in peripheral nerve repair[J].J Peripher Nerv Syst,2007,12:65-82.
[10]Evans GR.Peripheral nerve injury:a review and approach to tissue engineered constructs[J].Anat Rec,2001,263:39-404.
[11]Hudson TW,Evans GR,Schmidt CE.Engineering strategies for peripheral nerve repair[J].Orthop Clin North Am,2000,31:485-498.
[12]Johnson EO,Soucacos PN.Nerve repair:experimental and clinical evaluation of biodegradable artifcial nerve guides[J].Injury,2008,39:S30-S36.
[13]Cunha C,Panseri S,Antonini S.Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration[J].Nanomedicine,2011,7:50-59.
[14]de Ruiter GC,Malessy MJ,Yaszemski MJ,et al.Designing ideal conduits for peripheral nerve repair[J].Neurosurg Focus,2009,26:E5.
[15]Jiang X,Lim SH,Mao H,et al.Current applications and future perspectives of artificial nerve conduits[J].Exp Neurol,2010,223:86-101.
[16]Daly W,Yao L,Zeugolis D,et al.A biomaterials approach to peripheral nerve regeneration:bridging the peripheral nerve gap and enhancing functional recovery[J].J R Soc Interface,2012,9:202-221.
[17]Hadlock T,Sundback C,Hunter D,et al.A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration[J].Tissue Eng,2000,6:119-127.
[18]Rutkowski GE,Miller CA,Jeftinija S,et al.Synergistic effects of micropatterned biodegradable conduits and Schwann cellson sciatic nerve regeneration[J].J Neural Eng,2004,1:151-157.
[19]Hsu SH,Lu PS,Ni HC,et al.Fabrication and evaluation of microgrooved polymers as peripheral nerve conduits[J].Biomed Microdevices,2007,9:665-674.
[20]Hsu SH,Su C,Chiu I,et al.A novel approach to align adult neural stem cells on micropatterned conduits for peripheral nerve regeneration: a feasibility study[J].Artifcial Organs,2009,33:26-35.
[21]Long Y,Zhang N,Huang Y,et al.Formation of highly aligned grooves on inner surface of semipermeable hollow fiber membrane for directional axonal outgrowth[J].J Manuf Sci E-T ASME,2007,130:021011-1.
[22]Mulder M.Basic principles of membrane technology[M].In:The Netherlands:Kluwer Academic Publishers,1996.
[23]Yin J.Fabrication of grooved hollow fiber membrane for nerve regeneration:process modeling and performance evaluation[D].Clemson:Clemson University,2011.
[24]Culfaz PZ,Wessling M,Lammertink RGH.Hollow fiber untrafltration membranes with microstructured inner skin[J].J Memb Sci,2011,369:221-227.
[25]Yin J,Coutris N,Huang Y.Experimental investigation of aligned groove formation on the inner surface of polyacrylonitrile hollow fber membrane[J].J Memb Sci,2012,394:57-68.
[CLC number]R197.39 [Document code]A
doi:10.3969/j.issn.1674-1633.2016.04.001
[Article ID]1674-1633(2016)04-0001-05
Correspondence to:J.Yin,School of Mechanical Engineering,Zhejiang University,866 Yuhangtang Road,Hangzhou 310058,Zhejiang Province,China.